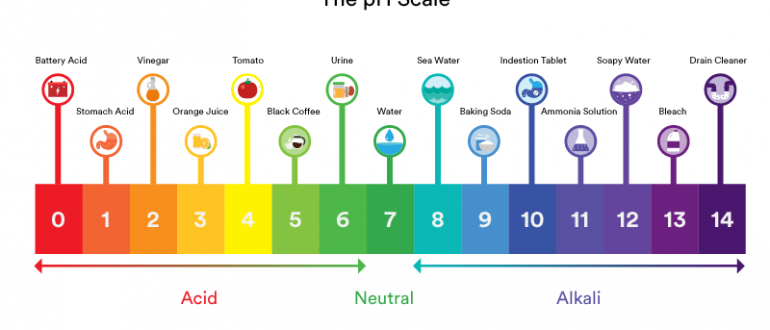
You have probably come across the word “pH” being used to describe the quality of water, but do you know what it means? It is certain that pH values are a good pointer of whether water is soft, hard or safe for drinking. So why does water pH seem to carry so much weight? Experts say that water pH is a strong pointer of how friendly the environment is.
pH Details
Water with a pH of 7 is regarded as pure or rather neutral. Water with a pH value lower than 7 is considered acidic while greater than 7 is considered to be alkaline. Basically, water pH is determined by the amount of hydrogen or hydroxyl ions found in water. Water with more hydrogen ions is passed as acidic while water with more hydroxyl ions is said to be alkaline. With the above details, we can certify that pH can be distorted by the chemicals that come into contact with water. Therefore, water pH is a vital indicator of water quality.
Importance Of Checking Water pH
Water pH varies from place to place depending on human activity, water storage, weather patterns, and other natural processes. For instance, water with a very high pH level may be a sign of heavy metal pollution or the presence of harmful chemicals. This, in turn, determines if aquatic life can thrive in the environment. With heavy metals present in water, the water may seem clean but the ions affecting aquatic life.
pH Tests
In this section, will take you back to your school days where one of the most popular chemistry tests was to test for acidity or alkalinity in different water samples. The most common test object used to test for water pH in normal settings is litmus paper. In advanced laboratories, chemists use complex electronic pH meter to test water pH. The litmus paper is a colored strip that tests pH when you dip it into a sample fluid. In this case, you have to roughly estimate the water pH depending on how the strip turns color.
What Water pH Level Is Safe For Drinking?
Experts say that water is considered safe for drinking if its pH levels fall between 6.5 and 8.5. Water beyond 8.5 in pH level is alkaline, which makes it unsafe for consumption. Excessively alkaline water may possess a bold unpleasant smell and salty taste when consumed. It may also destroy water pipes and other water storage containers.
On the other hand, water with pH levels of less than 6.5 is said to be acidic and more likely to be from a contaminated source. Contamination makes it unsafe for consumption and also causes corrosion on metal pipes. Water suppliers make pH testing a regular practice to help monitor for pollutants. If the pH value is not within the acceptable range, they take action to restore purity and eliminate any harmful chemicals.
Water purity revolves around water pH levels, which makes pH testing a common practice by water management bodies.